Activated carbon Adsorption to eliminate the impact of iron on the environment
Industrialization has promoted economic and social development, but the growing industrial facilities have aggravated pollutant emissions and affected the entire ecosystem. Water pollution is one of the distressing effects of industrialization. Data shows that 60% Surface water and 50% The iron ion contained in the drinking water of, due to mining activities, treatment plants and other activities, the iron content in the water source in some areas has increased greatly, and excessive iron accumulation may lead to serious health problems. In this study, the activated carbon adsorption technology was used to treat iron in water. In the experiment, the feasibility of removing iron ions by batch and fixed bed adsorption was studied.
Basic introduction of iron
No twenty-six Iron is an important element. It covers about 5% The earth's crust is the second most abundant metal, which is second only to oxygen, silicon and aluminum in the elements. Iron is a transition metal. The basic oxidation state is +2( ferrous ) and +3( Ferric iron ) , although there are +4( Ferrous group ) and +6( Ferrate ) Oxidized state. +2 The iron compound in the state of is labeled as ferrous, and is composed of light green Fe Ionic composition, and +3 An iron compound in the state of is labeled as ferric and contains Fe Complex ion, which changes from yellow to orange and finally to brown, depending on the degree of hydrolysis. In oxygenated water, Fe2+ Ions are oxidized to Fe3+ Ions. Oxidation rate mainly depends on H+ Ion concentration and solution temperature. Compared with ferric ion, ferrous ion usually retains more iron in solution, and ferrous element often has higher solubility compared with iron element.
Iron removal by adsorption
Iron is highly toxic, so it is necessary to control this pollutant at the emission level. There are many technologies that can be used to remove iron from drinking water and municipal waste, such as ion exchange and water softening, supercritical fluid extraction, aeration oxidation, and microfiltration / Ultrafiltration, aeration particle filter and bioremediation. The main disadvantage of these technologies is that they are either expensive or cannot be used without electricity. Adsorption method is to adsorb substances ( Adsorbate ) Adhere to solid surfaces. Adsorbents exist in the fluid phase as solutes dissolved in liquids or gases. Because of its ease of use, simple design, strong ability, low cost, low by-product production and good therapeutic effect, it is more popular than other treatment methods. This is a surface phenomenon, which requires pollutants in wastewater to gather on the surface of adsorbent. This deposition of metal ions on the adsorbent occurs at the interface of the adsorbent, resulting in a two-dimensional structure. This depends on the characteristics of the adsorbent, such as surface charge, surface area and surface function. Adsorption is also superior to other removal processes because of its high efficiency and the possibility of regenerating adsorbent and recovering adsorbent. One of the ways to overcome these difficulties is to adsorb iron through various adsorbents. Due to its multifunctional behavior, activated carbon has been determined to have high activity in wastewater treatment.
Activated carbon structure
The specific atomic configuration of activated carbon is not clear, although it has important commercial significance in water and air purification. Configuration of carbon prepared by pyrolysis. She pointed out that the carbon can be divided into two different groups, namely graphitization and non graphitization. The carbon derived from activated carbon is non graphitized, which means that even at very high temperatures (≥3000℃) It can also be transformed into crystalline graphite. Neutron diffraction studies show that the non graphitized carbon has a skeleton similar to fullerene, as shown in Figure one As shown in. This configuration will clarify the microporous structure of carbon and its other characteristics. from x The diffraction data obtained in X-ray and neutron diffraction studies have been clarified according to the structure composed of non hexagonal rings, but no clear evidence can be provided to show that atoms are bonded in the form of pentagonal rings or hexagonal rings.
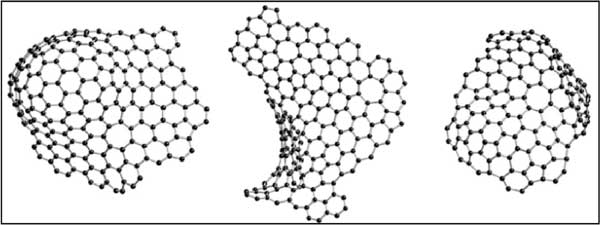
chart one : Schematic diagram of bending clip, including pentagon, heptagonal ring and hexagon.
In addition to the crystallization and chemical configuration of activated carbon, its porous structure also plays a vital role in various applications. The absorption capacity of activated carbon is highly related to its surface area, pore volume and pore size distribution. It mainly depends on the nature of raw materials and chemical treatment. The porous structure of activated carbon is produced by eliminating non carbon substances in raw materials during carbonization. The raw materials produce fixed carbon blocks with basic pore structure and further develop in the activation process. The activation process enlarges the diameter of the pores generated in the carbonization process, and some new pores are generated, thus forming a developed porous structure. The pores in activated carbon are distributed in various sizes and shapes. The pore size distribution of the obtained activated carbon is mainly affected by the degree of impregnation. The activation and carbonization conditions are the key parameters affecting the porosity types of the activated carbon obtained. The pore size of activated carbon is classified as follows: macropore (>50nm) , middle hole (2–5nm) And micropores (<2nm) 。 Micropores can be further subdivided into ultramicropores (<0.5nm) And ultramicropore (1-2nm) 。 The mesopore serves as the channel for adsorbents to pass through the microporous network. Macropores are meaningless, but they help guide metal ions into mesoporous and microporous surfaces.
Effect of actual working conditions on activated carbon adsorption of iron
The adsorption process depends to a large extent on various limiting conditions, such as pH Value, adsorbent dosage, initial concentration, contact time and temperature. The effects of these parameters on the adsorption of iron on activated carbon were studied in detail to determine the optimal conditions.
Activated carbon is used in two reach six Several of pH Excluded from the aqueous phase within the range Fe(II) Ions. They found that pH<3 The removal efficiency is low. With the solution pH When the value increases, the removal percentage increases significantly, and pH5.0 A large removal rate is reached when pH Value, H+ There are fewer and larger ones. The negatively charged ligand leads to higher metal adsorption. Heavy metal removal pH value 5-6 It is kept constant under, but due to the precipitation of metal hydroxide pH value six The above research.
The amount of iron adsorbed on the activated carbon increases with the increase of contact time. Due to the high driving force between adsorbent and metal ions in solution no The adsorption rate is at ninety Very fast in minutes. The accessible adsorption sites at the beginning slow down and one hundred and fifty Balance is reached in about minutes. Extend run time to six H has no effect on the residual concentration of metal ions, indicating that one hundred and fifty It reaches equilibrium in minutes. After this time, the accumulation of iron ions
