For the control of urban rainwater, it is necessary to consider the quantity and quality of water collected by underground rainwater collection pipes. This article focuses on Activated carbon Impact on rainwater treatment process in infiltration system. choice Activated carbon The selective removal of inorganic pollutants is studied by comparing with other treatment materials such as zeolite, limestone sand, diatomite and halloysite. The adsorption capacity of activated carbon was tested in batches under different environmental temperatures.
Urban rainwater contains a large number of pollutants, including suspension, oil derived substances, heavy metals, dissolved salts, polycyclic aromatic hydrocarbons, phenols and so on. In many areas, urban rainwater is regarded as one of the pollutants in the soil and water environment. Therefore, the collected rainwater needs to be treated before use or discharged into the water circulation system. The main purpose of this study is to determine the qualitative and quantitative impact of activated carbon adsorption in five different reactions. Activated carbon is used to measure the removal rate of pollutants in rainwater at low and full concentrations.
Treatment materials used
Activated carbon and unmodified mineral materials such as zeolite, limestone sand, diatomite and halloysite are used in the analysis (Fig. 1). Mineral materials are not subject to pretreatment to ensure the use of economically advantageous materials. The activated carbon used in this phase is a microporous material, and the surface of this material is proved by microporous surface and volume values and relatively low mesoporous surface values. Diatomite, zeolite and halloysite are mesoporous materials, so they are characterized by having the average specific surface area of the mesopore, and the mesopore has a narrow crack shape with irregular size and shape. The large specific surface area and volume of these mineral mesopores are observed in halloysite, while the pores of diatomite are relatively small. Limestone sand can be classified as non porous materials.

Figure 1: Reactive materials used in the study.
Scanning electron microscope (SEM) images and chemical micro range analysis (EDS) of reaction materials are shown in Figure 2. It is worth noting that the activated carbon sample has a large expanded porous surface, and the size of the internal pores can reach tens to hundreds of microns. SEM analysis of limestone sand confirmed the carbonate characteristics of this material, while micro chemical analysis of calcium and oxygen pointed to calcite (CaCO 3). SEM-EDS analysis shows that there are minerals from silica groups in diatomite samples, and it is allowed to classify the lithofacies of this material as diatomite. SEM micrographs confirm the surface of moderately developed zeolite and halloysite, and in the case of the latter material, there are characteristic nanotubes, however, they are not very obvious at this magnification. In addition, EDS spectra indicate that there are: silicon (Si), oxygen (O), aluminum (Al), potassium (K), calcium (Ca) and iron (Fe) in zeolite samples, as well as iron (Fe), aluminum (Al), silicon (Si), magnesium (Mg), phosphorus (P), calcium (Ca) and titanium (Ti) in halloysite samples.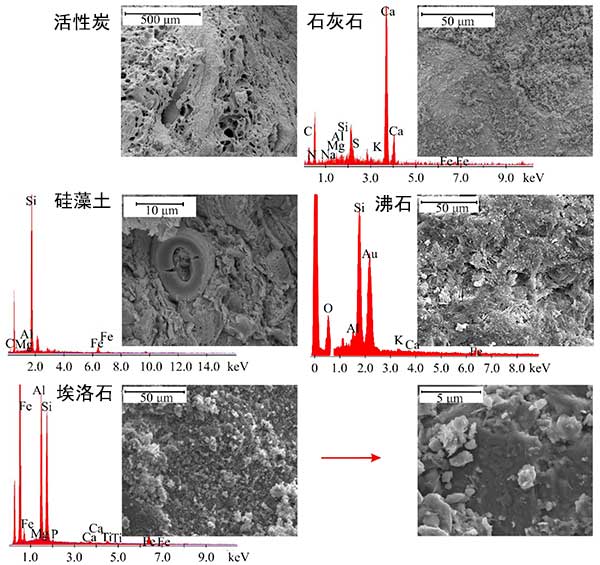
Figure 2. SEM images and EDS analysis of activated carbon and other materials.
Influence of external environment on removal effect
Activated carbon plays an important role in removing pollutants, especially heavy metal pollution, as well as ammonium ion. When the pH of water exceeds 7, in addition to ammonium ion, the water also contains gaseous ammonia, and when pH=11.1, the free ammonia reaches 99%. In the solution with higher pH value, heavy metals precipitate in the form of hydroxide. Conversely, in Pb with pH<7, Cu with pH<7.5, Zn with pH<8.7 and Ni with pH<9.8, the main metal form in rainwater inorganic matter treatment is their ionic form, which can be removed by activated carbon adsorption process. Therefore, it can be assumed that in the case of water containing Zn and Cu, activated carbon and limestone sand, due to their high pH values, precipitation will play an important role in removing these pollutants.
Effect of temperature on activated carbon adsorption at low initial concentration
Under the condition of empirical fitting, the change of equilibrium adsorption capacity of activated carbon for ammonium ion and phosphate in the temperature range of 3 ° C to 30 ° C is shown in Figure 3. For the biological components in rainwater, the test results were not conducted at 40 ℃, because the difference of the equilibrium concentration of the three repeated measurements was more than 10%. At the initial concentration of 10 mg/L NH 4-N and PO 4-P pollutants, no significant effect of temperature on the removal intensity of biological components was observed. The adsorption capacity of activated carbon relative to ammonium ion increases with the temperature changing from 3 ° C to 22 ° C. However, there is little difference between the total amount of ammonium ions removed by activated carbon at 3 ℃ and 22 ℃, while NH 4 reduces the removal of - N observed in the case of residual activated carbon.
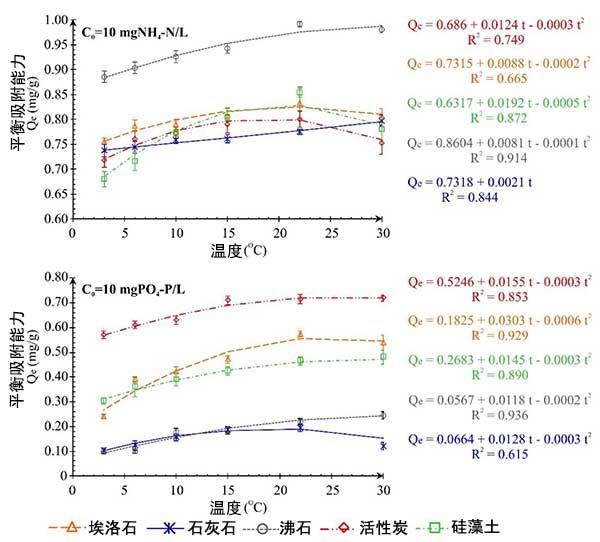
Fig. 3: Effect of temperature on inorganic pollution removal efficiency of activated carbon.
The balance test of ammonium and phosphate ion was only conducted in the temperature range of 3 ℃ to 22 ℃, so the relationship between adsorption capacity and temperature was not described by polynomial function. Similarly, for example, in the analysis of series I, activated carbon has a good effect on removing ammonium ions. In the case of phosphate, the material with high removal capacity is activated carbon.
This study emphasized the effect of temperature on the removal efficiency of typical selective inorganic pollutants from rainwater on activated carbon and low-cost mineral adsorbent. The investigation shows that the environmental temperature determines the condition of the underground soil layer. Therefore, in the underground infiltration treatment area, the removal of pollutants transported by rainwater in urban areas may be affected. With the decrease of temperature, the adsorption efficiency of activated carbon becomes weaker. However, the active position on the surface of activated carbon and the presence of other dissolved components of organic matter and suspension may affect the actual adsorption capacity of the material.
The above research shows that the material with high removal efficiency of Cu and PO 4 - P is activated carbon. The other materials are the medium efficiency of heavy metal removal and the low efficiency of biological base removal from the single component solution. Based on the value of large adsorption capacity, the treatment process is limited by temperature drop when Cu is removed on activated carbon and Zn is removed on limestone sand. In essence, it is beneficial to the removal process of activated carbon, which is nearly irreversible for high concentration of heavy metals, but not favorable for low concentration of phosphoric acid.


