Wearing masks in public places has become one of the effective protective measures when large-scale epidemic virus diseases spread around the world. Although masks are known to filter viruses, dust and harmful gases Activated carbon Layer mask may improve filtering efficiency. Although these masks are usually adequate, they may have limitations in protecting against certain gases, toxic vapors, and very small pathogens at a high respiratory rate. The antibacterial efficiency of activated carbon fiber is more than 90%. It is found that the electrostatic attraction between the virus and the carbon structure will cause the activated carbon to adsorb the virus. Generally, activated carbon has a high surface area to effectively remove viruses, bacteria, VOC, odor and other gaseous pollutants from the air. Active carbon with 20 – 50nm pores can effectively absorb viruses. The addition of active carbon layer has improved the protective ability of respirators by several grades.
Activated carbon The pore structure and carbon percentage of the adsorbent are directly related to its adsorption performance and application. Therefore, it is necessary to select the appropriate pore size of activated carbon for the correct application. For example, if the activated carbon sample has a microporous structure (<2nm), it will not be able to effectively adsorb gas molecules with mesopore size (2-50nm), such as some virus gas molecules, which are about 60-140nm. In order to accurately determine the adsorption capacity of activated carbon, it is necessary to study its isotherm, surface area and pore size distribution. Some commercial masks have produced filter bags with replaceable filters, which contain activated carbon layer inserts. Although some studies have been carried out on the filtration efficiency of masks and the influence of pore morphology, size and distribution on adsorption materials, the research on the surface characteristics of replaceable filter elements of masks for virus removal is limited. Therefore, this study aims to determine whether the commercial respirator with activated carbon is better than the ordinary respirator by focusing on the physical adsorption efficiency of the activated carbon layer purchased from multiple manufacturers in the commercial replaceable filter element.
Surface characteristics
It is necessary to analyze the surface area of adsorbent, because its adsorption capacity depends on it to a large extent. In order to obtain key information about the adsorption process, N2 adsorption isotherms are usually used to determine the surface area, pore volume and pore size distribution of the adsorbent. It is usually used for surface analysis. It is necessary to analyze the pore size distribution of activated carbon, because the difference of pore size will affect the adsorption capacity. For this purpose, N2 uses an instrument to analyze the adsorption isotherm, Brunauer, Emmett and Teller (BET) surface areas, and the pore size distribution of the adsorbent. Before N2 analysis, calculate the dry mass of the sample by subtracting the weight of the empty sample cell from the weight of the sample cell. Each sample was regenerated at 120 ℃ for more than 12 hours, and then the in-situ degassing process was carried out at the same temperature for 1 hour to successfully remove the adsorbed pollutants. Subsequently, density functional theory (DFT) was used to estimate the pore size distribution of each sample, and each sample was analyzed three times (n=3). This study tested four filter elements with activated carbon mask (as shown in Figure 1). As a result, Filter 1 has a very different appearance than the other three.

Figure 1: Four types of mask liners with activated carbon layers were used in this study. Each picture represents a different filter element.
adsorption isotherm
The adsorption isotherm of activated carbon has been studied using nitrogen (N2) adsorption analysis technology (a standard method). Figure 2 shows the N2 adsorption isotherm of activated carbon at 77K, which is found to be Type I isotherm. The rapid rise of N2 adsorption in the low relative pressure range confirmed the presence of microspores in all samples, as shown in Figure 2. It is obvious that activated carbon 4 has very low N2 adsorption, while the adsorption isotherms of activated carbon 1 and activated carbon 2 overlap, and activated carbon 3 has high N2 adsorption. This variability in data may be attributed to the different precursor materials used. In addition, except for activated carbon 3, most of the pore volume of activated carbon is filled below P/Po ≈ 0.1. For activated carbon 3, the pore volume filling is lower than P/Po ≈ 0.3. After the initial increase to 0.1, the isotherm curves gradually, indicating that the increment of further adsorption is small. With the increase of relative pressure, the adsorption of all samples is almost horizontal, indicating that they have reached saturation.
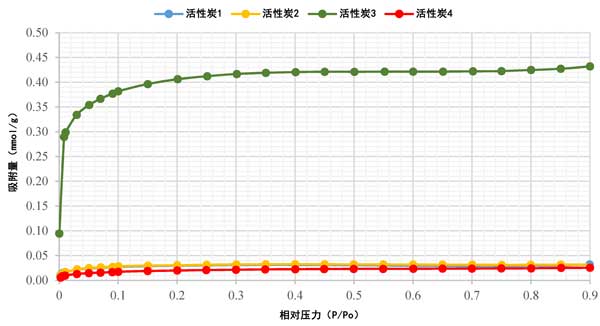
Figure 2: N2 adsorption isotherm of activated carbon.
Scanning electron microscope (SEM) analysis
At 1000 times magnification, individual fibers are clearly visible, and their cylindrical and smooth surfaces can be observed (Figure 3). The porosity of the samples was not observed in this study, which may be due to the small surface area of the samples, which makes them difficult to detect. In addition, activated carbon is not entirely made of carbon. As shown in the figure, the carbon layer is covered or sprayed by other materials. This may further lead to a low surface area and therefore a lack of observation of porosity.

Figure 3: SEM image of activated carbon layer magnified by 1000 times.
Adsorption molecules are captured on the surface of activated carbon, and higher fixed carbon content indicates a larger carbon surface area. Therefore, the fixed carbon content is the main factor for rough analysis. Typical activated carbon needs to have low ash content (2 – 10%), low moisture content (5 – 8%), and high carbon content (85 – 90%). Although there are some standards for volatile content of activated carbon in the form of powder (25%) and particle (15%), no standards are available in the literature. However, because ash and volatile substances will fill the pores of activated carbon, the high volatile content is not ideal, resulting in a reduction in surface area, which will adversely affect the adsorption performance of activated carbon.
The physical adsorption performance of the activated carbon layer in the mask. All samples have similar physical adsorption characteristics. Compared with other samples, the BET surface area of activated carbon 3 is slightly better, and the carbon content is higher than other samples. However, despite their physical properties, all samples have poor adsorption properties. Activated carbon 3 has two activated carbon layers, but because of its small surface area, it is not necessarily effective. In order to effectively filter viruses, it is recommended to use activated carbon with high surface area (>1000), high carbon percentage (>80%) and 0.1 micron pore size. The non-woven surface morphology of all samples has been observed. This study only focuses on the activated carbon of the mask filter element, and concludes that the filter element with activated carbon does not perform better in adsorption than the filter element without activated carbon. In addition, it is revealed that the insert is not a pure carbon material, and the polymer composition may dominate.


