Mechanical ball milling is a common material preparation technology, and also a post-treatment method to modify electrode materials to improve electrochemical performance. This research explores the impact of mechanical ball milling on business Activated carbon The effect on increasing the energy storage of sodium ions was proved by the modification of its microstructure. The evolution of physical and chemical properties of activated carbon during ball milling was systematically studied. It was observed that the number of closed pores and graphitization degree in activated carbon increased with the prolongation of ball milling time. The sodium storage mechanism in the activated carbon is transformed into the process of inserting pore filling, which significantly improves the platform capacity.
Closed pore engineering is usually realized by precursor selection, carbonization process control or chemical vapor deposition. Using ethanol as pore forming agent, closed pore structure was constructed in carbon materials to enhance sodium storage capacity. The reagent generates steam in the solvothermal process, leading to the formation of cavities between cross-linked matrices, which turn into closed pores during carbonization. The raw materials are carbonized at high temperature. This process promotes the reorganization of pre-existing open pores and disordered carbon structures, and generates a large number of closed nanoscale pores in the carbon framework. A space closed chemical vapor deposition technology was created, which integrates the graphite like carbon domain into the micropores of activated carbon. This process produces hard carbon materials with adjustable microstructure. However, these methods usually lack the accurate Therefore, a simple and controllable method is crucial for the preparation of high-capacity carbon anode in sodium ion batteries.
Material properties
Activated carbon Its precursor is coconut shell, which is widely used in supercapacitors and secondary batteries. Using a scanning electron microscope (SEM) To check the form of activated carbon material. chart 1a It is shown that the activated carbon without ball milling presents block shape, irregular shape and relatively loose particles. The ball milling process causes the activated carbon to be broken. With the increase of milling time, the particles become more spherical and the edges become rounded ( chart 1b) 。 This change may indicate a decrease in surface area. With the extension of ball milling time, the particles become more compact and finally gather ( chart 1f) , which may extend the ion transmission distance and reduce the magnification performance. HRTEM image ( chart S2) The evolution of graphite structure in activated carbon is clearly explained. The activated carbon shows a highly disordered structure, and the graphite area in the bulk phase very Less. In the sample, small graphite domains are formed due to mechanical milling. Then, through the growth and merging of these small areas, large areas of graphite are formed, accompanied by the elimination of nano pores. The above results show that ball milling, as a direct defect engineering method, is easily damaged due to strong impact and shear force C=C/CC Bond, leading to the rearrangement of carbon atoms, transforming the disordered structure into graphite structure. In addition, the importance of selecting proper grinding duration to process activated carbon was emphasized. It is worth noting that high temperature can promote the repair of defects and the elimination of oxygen functional groups. TEM The results show that the graphitization degree of the material is significantly improved after ball milling and secondary heat treatment.
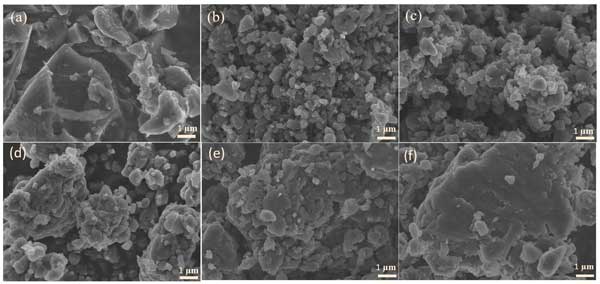
chart one : (a) Activated carbon (b) grind twelve Hours (c) grind twenty-four Hours (d) grind forty-eight Hours (e) grind sixty Hours and (f) grind seventy-two Hour SEM Image.
Nitrogen adsorption isotherm was used to check the specific surface area and pore structure of activated carbon. chart 2a Display, activated carbon displays IN2 Type adsorption - Desorption isotherm, characterized by low relative pressure (<0.1) And reach a stable state in the area from medium pressure to high pressure. The pattern shows that it is mainly microporous structure, with a small amount of narrow mesopores ( chart 2b) 。 After mechanical ball milling, with the increase of ball milling time, N2 The adsorption capacity gradually weakens, sixty Hours later, it is close to zero. This trend indicates the elimination of open pores. Therefore, the specific surface area and pore volume of activated carbon begin to decline ( chart 2c) 。 In order to further study the change of pore structure, closed pore test was carried out. With the increase of milling time, the closed pore volume increases ( chart 2d) 。 Grinding with activated carbon sixty The specific surface area and pore volume of the activated carbon sample after heat treatment increased slightly compared with that of the sample after heat treatment, because some micropores were generated by removing gas from the bulk phase during the heat treatment process. However, the closed pore content remained almost unchanged. These changes in pore structure show that good milling duration is an effective method to reduce the specific surface area of activated carbon and increase the closed pore volume.
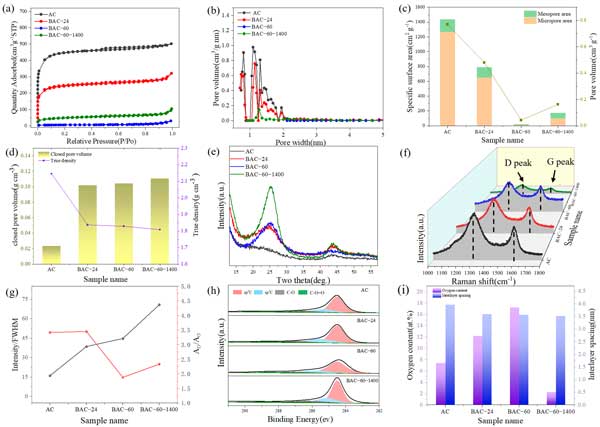
chart two : Structure characterization of ball milled activated carbon. (a)N2 adsorbent - Desorption isotherm ; (b) Pore size distribution ; (c) Specific surface area and pore volume ; (d) Closed cell volume and true density ; (e)XRD atlas ; (f) Raman spectra ; (g) Raman spectroscopic XRD(002) Change of peak intensity ; (h)XPSC1s Spectrum ; (i) Oxygen content and layer spacing.
chart 2e Shows the XRD Spectrum. about 22° and 44° At (002) and (100) The diffraction peak intensity is low and wide, indicating that the structure is highly disordered. As the ball milling time is extended to twenty-four These peaks gradually sharpen and strengthen when. (002) The peak moves to a higher angle, about 26° , indicating that after ball milling d002 Increase in layer spacing ( chart 2i) 。 Microcrystalline size, especially transverse size (La) And stack height (Lc) , is using Debye-Scherrer Equation. Activated carbon, grinding twenty-four Hours and sixty Hour Lc(002) The values are two point seven three 、 two point four nine and 2.46nm Of these samples La(100) The measured values are one point four one five 、 one point four two two and 1.432nm 。 To illustrate the change in crystallinity, use (002) Peak intensity and full width at half peak (FWHM) Ratio of ( chart 2g) 。 The ratio varies from sixteen point one Monotonic increase to grinding sixty Hour forty-four point seven It shows that the graphitization degree of activated carbon is significantly improved. Change of graphitization degree and TEM agreement.
Similarly, Raman spectrum ( chart 2f) It shows that with the increase of ball milling time of activated carbon, D Band and G Strength ratio of belt (ID/IG) Lower. It comes from activated carbon one point four zero Lower to grinding sixty Hours later one point one five , indicating that the carbon structure is more orderly. As shown in the figure 2g As shown in, D Band and G Area ratio of belt (AD/AG) Initially from activated carbon three point four two Add to grinding twenty-four Hours later three point four five And then it drops sharply to activated carbon grinding sixty Hours later one point eight eight 。 The initial increase in defect density is attributed to the decomposition of activated carbon particles, which reveal pores and edges. The subsequent decline in the ratio indicates that “ Destructive ” Ball miller

