
Honeycomb zeolite molecular sieve is a kind of aluminosilicate material containing alkali metal or alkaline earth metal oxide with open framework and regular pore cage structure. It has been widely used since it was discovered by Swedish scientists in 1956, and then it has been widely used in petrochemical industry, environmental protection, bioengineering and other fields due to its special physical characteristics. high quality And with the gradual expansion of the demand for zeolite molecular sieves, researchers began to expand a variety of synthetic methods of zeolite molecular sieves to meet the needs of various fields. Zeolite molecular sieve has super strong adsorption performance, which is due to a "surface force" generated by molecular gravity on the solid surface. When the fluid flows, some molecules in the fluid collide with the adsorbent surface due to irregular movement, resulting in molecular concentration on the surface, reducing the number of such molecules in the fluid to separation Purposes of clearing. production Since the adsorption does not undergo chemical changes, as long as the molecules concentrated on the surface are managed to drive away, the zeolite molecular sieve will have the adsorption capacity again. This process is the reverse process of adsorption, which is called the resolution or regeneration process.

high quality The addition of retention aids in the wet end of paper making is an important way to improve filler retention, which has been widely used in industrial production. Some studies reported that clinoptilolite (particle size 0.5-2 μ m) was used as a microparticle retention aid to compare the retention effect with silica and bentonite microparticle retention aid systems. The retention effect of clinoptilolite retention aid is equivalent to that of the silica microparticle retention aid system with the same dosage, which is far better than that of the bentonite microparticle retention aid system. And the use of zeolite as paper filler eliminates the need for additional microparticle retention aids. It is also reported that the retention rate of TiO2 nanoparticles can be significantly improved by using zeolite molecular sieves as retention aids in the preparation of photocatalytic paper. Compared with starch and cationic polymer, zeolite molecular sieve microparticle retention aids have better effects on improving the retention and drainage properties of paper stock and improving the uniformity of paper. high quality The use of zeolite particle retention aids can effectively avoid flocculation between fibers, and help to form fiber particle fiber flocculating particles. In addition, it can also support titanium dioxide nanoparticles.
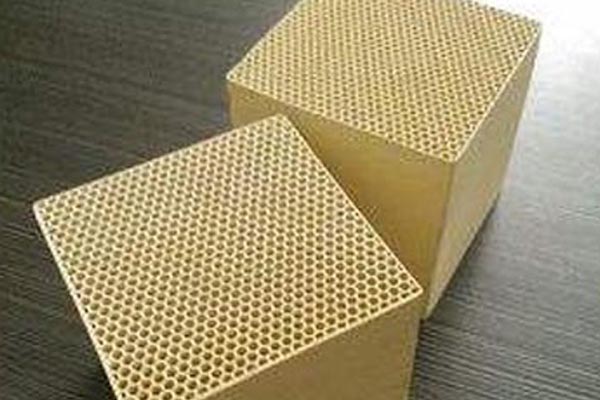
Because there are uniform small internal pores in the molecular sieve structure, Hebei When the molecular linear degree of reactants and products is close to the pore size of the crystal, the selectivity of catalytic reaction often depends on the corresponding size of the molecule and pore size. This selectivity is called shape selective catalysis. There are two mechanisms leading to shape selectivity. One is caused by the difference of diffusion coefficient of molecules participating in the reaction in the pore cavity, which is called mass transfer selectivity; The other is caused by the space limitation of the transition state of the catalytic reaction, which is called transition state selectivity. Molecular sieve has clear pore cavity distribution, extremely high internal surface area (600m2/s), good thermal stability (1000 ℃), and adjustable acid site center. The acidity of molecular sieve mainly comes from three coordinated aluminum atoms and aluminum ions (AlO)+on the framework and in the pores. The OH based acid sensitive site center on the molecular sieve HY obtained by ion exchange, and the aluminum ion outside the framework will strengthen the acid site, forming the L acid site center. Polyvalent cations such as Ca2+, Mg2+and La3+can show the acid site center after exchange. The reduction of transition metal ions such as Cu2+and Ag+can also form acid site centers. In general, the higher the Al/Si ratio, the higher the specific activity of OH group. The modification of zeolite acidity can introduce protons through direct exchange of dilute hydrochloric acid. high quality This method often leads to dealumination of molecular sieve framework. So NaY will become NH4Y and then HY.
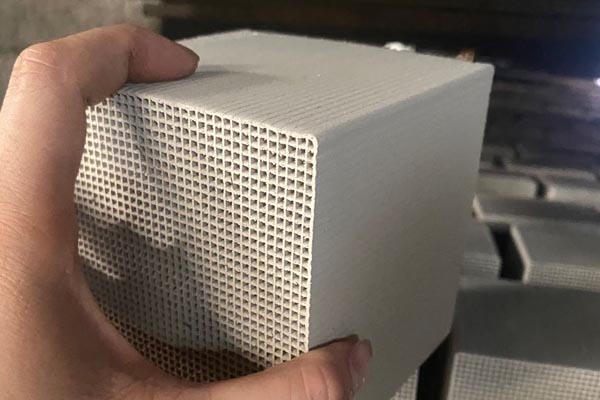
high quality The evaluation of the adsorption of zeolite depends not only on whether the specific surface area of zeolite is large enough, but also on whether the equipment is hydrophobic. When purchasing, people can choose the inspection equipment. When the air humidity for the hydrophobicity of the inspection equipment is greater than 60%, if the equipment is blocked and the machine works abnormally, people should carefully consider whether to purchase the equipment. It is important to know that the adsorption and hydrophobicity of the zeolite runner are equally important. The zeolite content of the zeolite runner is proportional to the adsorption concentration efficiency. Therefore, many people should pay more attention to whether the zeolite content of the equipment meets the requirements or is high enough when choosing to purchase the zeolite runner. On the one hand, selecting excellent equipment is also to enhance the potential value of the enterprise. Hebei The adsorption and concentration technology of zeolite runner is deeply loved by enterprises because of its high efficiency and energy saving characteristics. Its changeable working environment and low cost have laid an unshakable position for zeolite runner in industry. How to select a zeolite runner with stable operation and high efficiency needs people's eyes
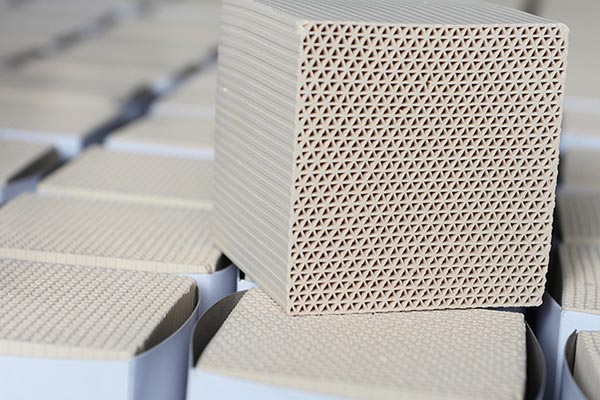
high quality The highly effective selective adsorption is due to the fact that the alumina tetrahedron of zeolite has a negative charge, while the skeleton hole contains cations, which forms a strong electric field around the cations. Therefore, the adsorption force of zeolite not only has a strong dispersion force, but also has a large electrostatic force. It is precisely because of this electrostatic relationship that zeolites have preferential selective adsorption on polar, unsaturated and polarizable molecules. It can strongly adsorb molecules containing polar groups such as hydroxyl ion, ammonium ion and other polarizable groups, especially water, which can form hydrogen bonds with aluminum silicon skeleton, so zeolite has strong water absorption. It can still adsorb even under low relative humidity and concentration, and its water absorption is higher than that of silica gel and activated alumina. The adsorption capacity of zeolite for organic pollutants mainly depends on the polar size and molecular diameter of organic molecules. Small molecules are easier to be adsorbed than large molecules, while polar molecules are easier to be adsorbed than non-polar ones. Different substances (such as organic molecules, metal ions and water molecules) exist in water, production Their polarity and molecular size are different, and competition will occur during adsorption.
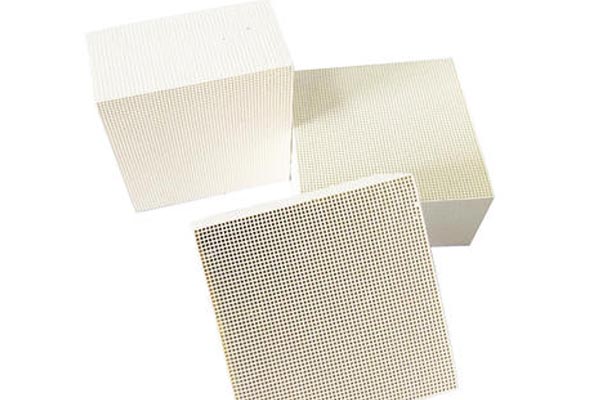
Hebei Since the adsorption does not change chemically, high quality As long as we try to drive away the molecules concentrated on the surface, zeolite molecular sieves will have adsorption capacity again. This process is the reverse process of adsorption, which is called the process of desorption or regeneration. Honeycomb zeolite molecular sieve is a kind of aluminosilicate material containing alkali metal or alkaline earth metal oxide with open framework and regular pore cage structure. It has been widely used since it was discovered by Swedish scientists in 1956, and then it has been widely used in petrochemical industry, environmental protection, bioengineering and other fields due to its special physical characteristics. And with the gradual expansion of the demand for zeolite molecular sieves, researchers began to expand a variety of synthetic methods of zeolite molecular sieves to meet the needs of various fields. Zeolite molecular sieve has super strong adsorption performance, which is due to a "surface force" generated by molecular gravity on the solid surface. When the fluid flows, some molecules in the fluid collide with the adsorbent surface due to irregular movement, resulting in molecular concentration on the surface, reducing the number of such molecules in the fluid to separation Purposes of clearing





