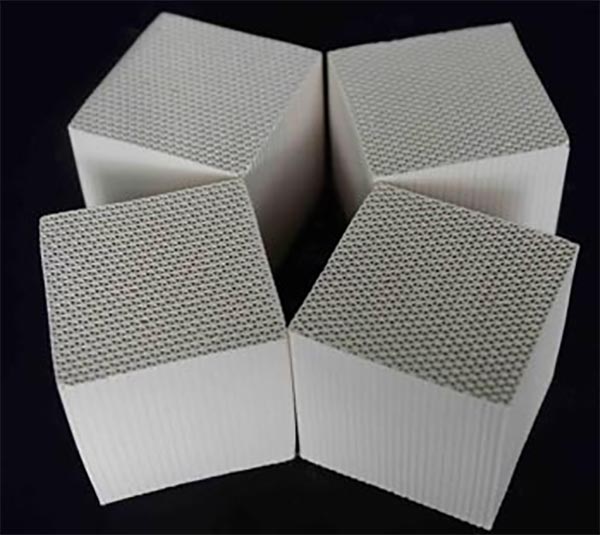
Honeycomb zeolite molecular sieve Its main material is natural zeolite, which is composed of SiO2 Inorganic microporous material composed of Al2O3 and alkaline metal or alkaline earth metal, with inner pore volume accounting for 40-50% of the total volume and specific surface area of 300-1000 m2/g, has the characteristics of high temperature resistance, non flammability, good thermal stability and hydrothermal stability. It is an efficient molecular sieve carrier with good adsorption performance, no secondary pollution, and can be regenerated at high temperature, which is better than similar carriers Activated carbon The efficiency is increased by 40%, which is widely used in the fields of adsorption, separation, catalysis and environment, and is more suitable for the treatment of organic waste gas with large air volume and low concentration. The adsorption material has a stable ozone decomposition ability, which significantly improves the decomposition efficiency of VOCs. The adsorption material first forms a special structural effect through the combination design of silver and manganese, which can decompose ozone into active oxygen atoms, and then react with VOCs molecules to form carbon dioxide and water.
Type A molecular sieve
Cubic crystal system structure similar to NaCl. If all Na+and Cl - in the NaCl lattice are replaced with β cages, and the adjacent β cages are connected with γ cages, the crystal structure of A-type molecular sieve can be obtained. Eight β cages are connected to form a sodalite structure. If γ cage is used as bridge connection, A-type molecular sieve structure will be obtained. There is a big α cage in the center. The channel between the α cages has an eight membered ring window with a diameter of 4 ∨, so it is called 4A molecular sieve.
If 70% of the sodium ion on 4A molecular sieve is exchanged by Ca2+, the eight membered ring can be increased to 5 ∨, and the corresponding zeolite is called 5A molecular sieve. Conversely, if 70% of Na+is K+exchanged, the pore size of the octagonal ring will be reduced to 3 ∨, and the corresponding zeolite is called 3A molecular sieve.
X-type and Y-type molecular sieves are similar to the compact hexagonal crystal system structure of diamond. If β cage is used as the structural unit to replace the carbon atom node of diamond, and two adjacent β cages are connected by hexagonal cylindrical cages, that is, five β cages are connected by four hexagonal cylindrical cages, one of which is in the center, and the other four β cages are located at the apex of the tetrahedron, the octahedral zeolite type crystal structure is formed.
If this structure continues to be linked, the X - and Y-type molecular sieve structures will be obtained. In this structure, the large cage formed by β cage and hexagonal column cage is octahedral zeolite cage, and the window hole they communicate is a twelve membered ring, with an average effective pore diameter of 0.74nm, which is the pore diameter of X-type and Y-type molecular sieves. The main difference between the two models is that the Si/Al ratio is different, and the X-type is 1~1.5; Y-type is 1.5~3.0.


